Conducting clinical trials is a crucial aspect of medical research, as it helps determine the safety and efficacy of new interventions, such as drugs, devices, or therapies, before they can be approved for widespread use. Designing a well-structured clinical trial study is essential to ensuring the validity and reliability of the research findings. This blog post will explore the key considerations and steps in designing a clinical trial study.
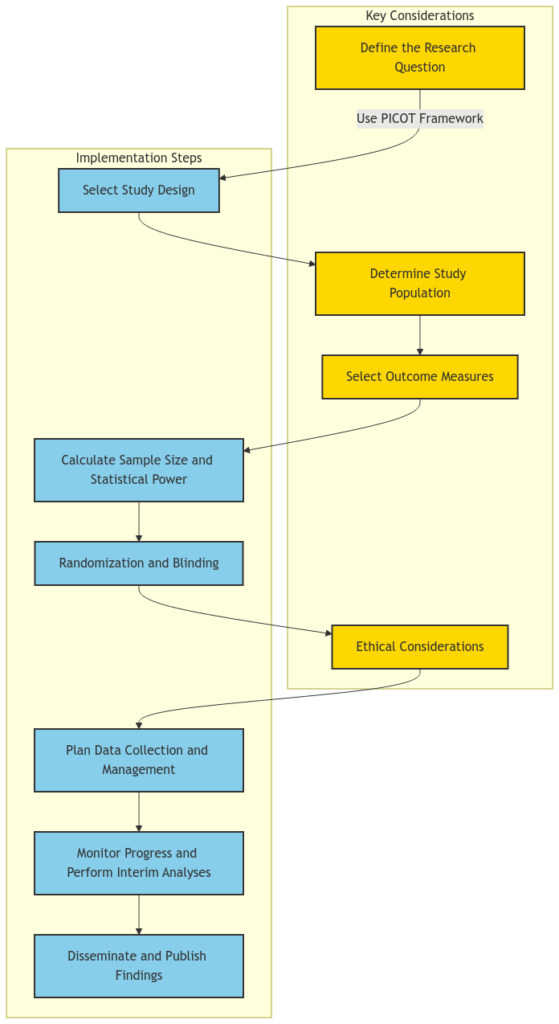
Defining the Research Question:
The first and most crucial step in designing a clinical trial study is to clearly define the research question. This involves identifying the target population, the intervention or treatment being investigated, the comparison group (if applicable), the outcome measures, and the time frame for the study. The PICOT (Population, Intervention, Comparison, Outcome, Time) framework is a widely used tool to help formulate a well-structured research question.
Selecting the Study Design:
The choice of study design depends on the research question and the available resources. Common study designs in clinical research include randomized controlled trials (RCTs), observational studies (e.g., cohort studies, case-control studies), and pragmatic trials. Each design has its own strengths and limitations, and the researcher must carefully consider the trade-offs between internal validity (the ability to establish a causal relationship) and external validity (the generalizability of the findings).
Defining the target population and the accessible population is crucial for the success of a clinical trial. The target population is the population of interest from which the researcher wants to draw conclusions, while the accessible population is the subset of the target population that is available for the study. Careful consideration of inclusion and exclusion criteria, as well as strategies for recruitment and retention, can help ensure the study population is representative of the target population.
The choice of outcome measures is critical in clinical trial design. Researchers must decide whether to use clinical outcomes (e.g., mortality, morbidity) or surrogate endpoints (e.g., biomarkers, laboratory values) as the primary outcome. Surrogate endpoints can be more practical and efficient, but they must be validated and have a clear relationship to the clinical outcome of interest. Additionally, researchers should consider the use of composite endpoints, which combine multiple outcomes into a single measure, to increase the statistical power of the study.
Calculating the appropriate sample size is essential to ensure the study has sufficient statistical power to detect a meaningful difference between the intervention and comparison groups. This calculation takes into account factors such as the expected effect size, the desired level of statistical significance, and the anticipated rate of dropout or loss to follow-up. Researchers should also consider the use of interim analyses and stopping rules to ensure the ethical and efficient conduct of the trial.
Randomization is a key feature of RCTs, as it helps to ensure that any observed differences between the intervention and comparison groups are due to the intervention itself, rather than confounding factors. Blinding, where participants, researchers, or outcome assessors are unaware of the assigned treatment, can also help reduce bias and strengthen the validity of the study findings. Researchers must carefully consider the feasibility and ethical implications of blinding in their study design.
Ethical principles, such as respect for persons, beneficence, and justice, must be at the forefront of clinical trial design. Researchers must ensure that the study protocol is reviewed and approved by an Institutional Review Board (IRB) or Ethics Committee, and that informed consent is obtained from all participants. Additionally, they must consider the potential risks and benefits to participants, as well as the fair selection of study participants.
Careful planning of data collection and management is essential to ensure the integrity and quality of the study data. This includes the development of data collection forms, the implementation of quality control measures, and the establishment of data management protocols. Researchers should also consider the use of electronic data capture systems and the secure storage and handling of sensitive participant information.
Ongoing monitoring of the study progress and safety of participants is crucial. Researchers should establish a data and safety monitoring board (DSMB) to regularly review the study data and make recommendations about the continuation, modification, or termination of the trial. Interim analyses can also be used to assess the feasibility and potential efficacy of the intervention, and to make adjustments to the study design if necessary.
The final step in the clinical trial design process is the dissemination and publication of the study findings. Researchers should plan for the timely and transparent reporting of the study results, following established guidelines such as the CONSORT (Consolidated Standards of Reporting Trials) statement. This helps ensure the study findings are accessible to the broader scientific community and can inform future research and clinical practice.
Designing a well-structured clinical trial study requires a comprehensive understanding of research methodology, statistical principles, and ethical considerations. By carefully addressing each of these key elements, researchers can increase the likelihood of conducting a successful and impactful clinical trial that advances medical knowledge and improves patient outcomes.


